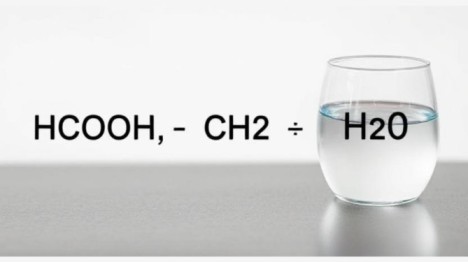
What Do HCOOH, CH₂, and H₂O Represent in Chemistry?
In chemistry, every formula tells a story. HCOOH represents formic acid, one of the simplest carboxylic acids. It is widely used in biological systems and industrial processes. CH₂ generally refers to a methylene group, a basic unit in organic chemistry that appears in chains and cyclic compounds. H₂O, the most familiar of the three, is water, the universal solvent essential to life.
When we put these together hcooch ch2 h2o we open a window into reaction pathways, organic mechanisms, and structural relationships that are fundamental to both theory and practice in chemistry. Understanding these elements individually and in combination can help explain why certain reactions occur the way they do.
Why Is Formic Acid (HCOOH) Important in Reactions?
Formic acid, or HCOOH, plays a critical role as both a reactant and a by-product in organic chemistry. Its simple structure makes it reactive under mild conditions. This allows it to participate in a wide variety of reactions such as esterification, reduction, and oxidation.
One of the unique aspects of HCOOH is that it can act as both a weak acid and a reducing agent. For instance, in some reactions, it donates a proton (H⁺) to stabilize intermediates. In other cases, it reduces metal complexes, contributing to catalytic cycles.
This dual role makes HCOOH a versatile compound when it interacts with groups like CH₂ in aqueous (H₂O) environments.
How Does the CH₂ Group Interact in Organic Chemistry?
The CH₂ group, or methylene, is a fundamental building block of organic molecules. It appears in countless compounds, from simple hydrocarbons to complex biological macromolecules. Its importance lies in its ability to connect carbon atoms, forming chains and branches that define molecular structures.
In reactions, CH₂ often acts as a reactive center. For example, in carbene chemistry, a CH₂ unit can exist in a highly reactive state, ready to form bonds with other atoms or groups. When water (H₂O) and formic acid (HCOOH) are present, CH₂-containing intermediates may stabilize or transform into more complex molecules.
This interplay highlights why chemists study combinations like hcooch ch2 h2o to better understand synthetic pathways.
Why Is Water (H₂O) Central to These Reactions?
Water is more than a solvent. It actively participates in many chemical processes. Because of its polarity, water can stabilize charged intermediates, making reactions more efficient. Additionally, water can provide or remove protons, influencing reaction pathways.
In the context of HCOOH and CH₂, H₂O often ensures that reactions proceed in a controlled manner. For example, hydrolysis reactions, which break chemical bonds using water, often involve formic acid derivatives. The outcome of hcooch ch2 h2o interactions may depend heavily on how water influences solubility, reactivity, and energy balance.
What Reactions Involve HCOOH, CH₂, and H₂O Together?
When we look at the trio HCOOH, CH₂, and H₂O several reaction types stand out:
- Hydration and Hydrolysis
CH₂ groups adjacent to reactive centers may undergo hydration in the presence of water, leading to alcohol formation. If formic acid is present, it may catalyze the process. - Ester Formation
HCOOH can react with alcohol groups generated from CH₂ hydration, forming esters. Water acts as both a medium and sometimes a by-product. - Oxidation Pathways
Under specific conditions, formic acid may oxidize methylene groups, with water stabilizing intermediates. - Carbene Insertion
CH₂ in carbene form may interact with HCOOH, leading to unique organic products in aqueous environments.
These examples illustrate how hcooch ch2 h2o combinations are not just theoretical but practical in laboratory and industrial chemistry.
How Is HCOOH + CH₂ + H₂O Used in Industry?
Industrial chemistry relies on efficiency, and the HCOOH–CH₂–H₂O system plays a part in several processes:
- Textile and Leather Treatment
Formic acid is used for tanning and fabric treatment. The presence of CH₂-based chemicals and water determines the stability and effectiveness of the reaction. - Fuel Cells
HCOOH is considered a potential hydrogen source in direct formic acid fuel cells (DFAFCs). CH₂ groups are important in fuel additives, and water ensures conductivity. - Polymer Production
CH₂ units are fundamental in polymer backbones. The presence of formic acid and water influences polymerization rates and product stability.
Understanding these uses gives real-world significance to studying hcooch ch2 h2o chemistry.
What Does the Mechanism of Interaction Look Like?
Chemists often ask: What is the step-by-step pathway when these compounds interact? While mechanisms can vary, a simplified version may look like this:
- Activation of HCOOH
Formic acid donates a proton to activate a functional group. - CH₂ Reaction Initiation
The methylene group bonds or rearranges under the acidic conditions. - Water’s Role
H₂O stabilizes intermediates or contributes to hydrolysis steps. - Product Formation
Depending on conditions, esters, alcohols, or other organic derivatives are formed.
The outcome depends on temperature, pH, catalysts, and concentrations.
How Does This Chemistry Relate to Biological Systems?
It might surprise you to know that hcooch ch2 h2o interactions are not just industrial. In biology, similar reactions happen in metabolic pathways.
Formic acid is a by-product in some organisms. Methylene groups appear in DNA synthesis, specifically in one-carbon metabolism. Water, of course, is the medium in which all these reactions occur.
Thus, studying these interactions in a lab helps scientists better understand natural biochemical processes.
What Are the Safety Considerations with HCOOH and CH₂?
Chemicals like HCOOH and reactive CH₂ intermediates require careful handling:
- HCOOH (Formic Acid): Corrosive and can cause burns on skin or eyes. Inhalation is dangerous.
- CH₂ Groups/Carbenes: Highly reactive, potentially explosive under uncontrolled conditions.
- H₂O (Water): While safe, its role in reactions may accelerate dangerous processes if not controlled.
Therefore, any study of hcooch ch2 h2o reactions must include strict laboratory protocols.
Frequently Asked Questions (FAQ)
Is HCOOH the same as HCOOCH?
No. HCOOH is formic acid, while HCOOCH could represent a formate ester. They are related but not identical.
Can CH₂ exist freely?
In normal conditions, CH₂ does not exist freely. It appears as part of larger molecules or as a short-lived reactive species (carbene).
Why is H₂O so important in these reactions?
Water stabilizes intermediates, participates in proton transfer, and allows reactions to proceed in an aqueous medium.
What happens when HCOOH reacts with CH₂ in water?
Depending on conditions, reactions may produce esters, alcohols, or other organic products. The exact mechanism depends on catalysts and energy input.
Is this chemistry only theoretical?
No. The combination of HCOOH, CH₂, and H₂O has real applications in fuel cells, textiles, polymers, and even biology.
Conclusion: Why Does Breaking Down HCOOH, CH₂, and H₂O Matter?
The study of hcooch ch2 h2o highlights how small molecules can play large roles in chemistry. Formic acid (HCOOH), methylene groups (CH₂), and water (H₂O) interact in ways that are relevant to laboratory research, industrial processes, and even biological systems.